Transportadores de Ca2+ y su papel en las características distintivas del cáncer
DOI:
https://doi.org/10.29059/cienciauat.v19i2.1917Palabras clave:
cáncer, transportadores de calcio, proliferación celular, apoptosis, migración e invasión celularResumen
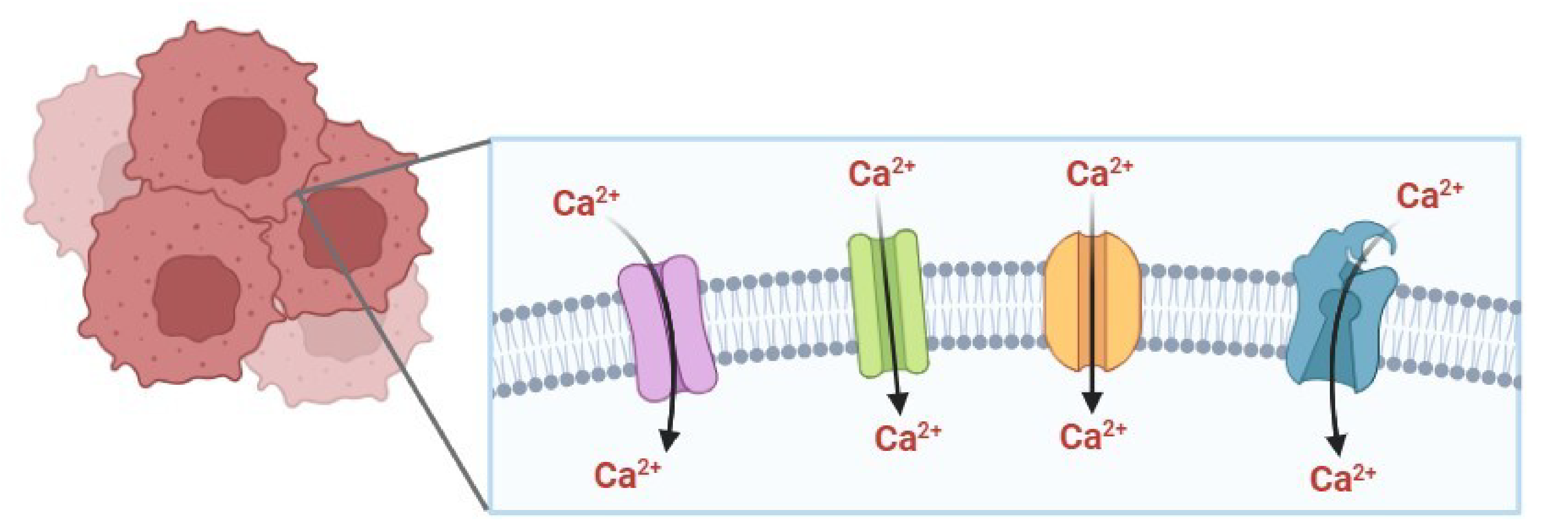
El ion calcio (Ca2+) activa diversas vías de señalización importantes en diferentes procesos celulares como proliferación, progresión del ciclo celular, apoptosis y expresión génica. La homeostasis de Ca2+ depende de diversas proteínas, que actúan como canales, bombas, receptores, sitios de unión y almacenamiento de Ca2+, las cuales son de gran importancia porque regulan el flujo, compartimentación y concentración del Ca2+ celular, para que las vías de señalización dependientes de este catión funcionen adecuadamente. El objetivo del presente trabajo fue analizar la información existente sobre los cambios en la expresión de transportadores de Ca2+ en cáncer y su participación en las características distintivas de la enfermedad, principalmente la proliferación celular descontrolada, la resistencia a la apoptosis o la activación de la migración e invasión celular. La evidencia indica que múltiples canales de Ca2+ se sobreexpresan en cáncer, lo que se asocia con incremento del Ca2+ citoplásmico y activación de las vías de señalización CaM/CaN/NFAT, Akt o MAPK/ERK, situación que puede conducir a un incremento en la proliferación, transición epitelio-mesenquimal, mayor capacidad de migración e invasión celular. Por otro lado, la subexpresión de bombas de Ca2+ o sobreexpresión de canales mitocondriales contribuye a la evasión de la apoptosis, a la par que propicia la migración celular. El estudio de transportadores de Ca2+ con expresión alterada en cáncer puede contribuir a la identificación de potenciales biomarcadores o blancos terapéuticos que permitan el desarrollo de nuevas terapias.
Citas
Almasi, S., Sterea, A. M., Wasundara, F., Clements, D. R., Marcato, P., Hoskin, D. W., Gujar, S., & El-Hiani, Y. (2019). TRPM2 ion channel promotes gastric cancer migration, invasion and tumor growth through the AKT signaling pathway. Scientific Reports, 9(1), 4182. https://doi.org/10.1038/S41598-019-40330-1 DOI: https://doi.org/10.1038/s41598-019-40330-1
Asghar, M. Y., Lassila, T., Paatero, I., Nguyen, V. D., Kronqvist, P., Zhang, J., Slita, A., Löf, C., Zhou, Y., Rosenholm, J., & Törnquist, K. (2021). Stromal interaction molecule 1 (STIM1) knock down attenuates invasion and proliferation and enhances the expression of thyroid-specific proteins in human follicular thyroid cancer cells. Cellular and Molecular Life Sciences, 78(15), 5827-5846. https://doi.org/10.1007/S00018-021-03880-0 DOI: https://doi.org/10.1007/s00018-021-03880-0
Ay, A. S., Benzerdjerb, N., Sevestre, H., Ahidouch, A., & Ouadid-Ahidouch, H. (2013). Orai3 constitutes a native store-operated calcium entry that regulates non small cell lung adenocarcinoma cell proliferation. PLoS One, 8(9), 72889. https://doi.org/10.1371/JOURNAL.PONE.0072889 DOI: https://doi.org/10.1371/journal.pone.0072889
Bahar, M. E., Kim, H. J., & Kim, D. R. (2023). Targeting the RAS/RAF/MAPK pathway for cancer therapy: from mechanism to clinical studies. Signal Transduction and Targeted Therapy, 8(1), 455. https://doi.org/10.1038/S41392-023-01705-Z DOI: https://doi.org/10.1038/s41392-023-01705-z
Berridge, M. J., Bootman, M. D., & Roderick, H. L. (2003). Calcium signalling: dynamics, homeostasis and remodelling. Nature Reviews. Molecular Cell Biology, 4(7), 517-529. https://doi.org/10.1038/nrm1155 DOI: https://doi.org/10.1038/nrm1155
Bouchard, M. J., Wang, Y., Deng, X., Zhang, R., Lyu, H., Xiao, S., Guo, D., Ali, D. W., Michalak, M., Zhou, C., Chen, X. Z., & Tang, J. (2024). The TRPV6 calcium channel and its relationship with cancer. Biology (Basel), 13(3), 168. https://doi.org/10.3390/BIOLOGY13030168 DOI: https://doi.org/10.3390/biology13030168
Cai, X., Yu, X., Yang, J., Lu, L., Hua, N., Duan, X., Ye, P., Ni, L., Jiang, L., Yang, W., Liang, T., & Yu, P. (2023). TRPM2 regulates cell cycle through the Ca2+-CaM-CaMKII signaling pathway to promote HCC. Hepatology Communications, 7(5), 1-15. https://doi.org/10.1097/HC9.0000000000000101 DOI: https://doi.org/10.1097/HC9.0000000000000101
Carafoli, E. & Krebs, J. (2016). Why calcium? How calcium became the best communicator. Journal of Biological Chemistry, 291(40), 20849-20857. https://doi.org/10.1074/jbc.R116.735894 DOI: https://doi.org/10.1074/jbc.R116.735894
Chen, T. M., Huang, C. M., Hsieh, M. S., Lin, C. S., Lee, W. H., Yeh, C. T., & Liu, S. C. (2022). TRPM7 via calcineurin/NFAT pathway mediates metastasis and chemotherapeutic resistance in head and neck squamous cell carcinoma. Aging (Albany NY), 14(12), 5250-5270. https://doi.org/10.18632/AGING.204154 DOI: https://doi.org/10.18632/aging.204154
Chen, Y. W., Chen, Y. F., Chiu, W. T., Chen, H. C., & Shen, M. R. (2017). STIM1-dependent Ca2+ signaling regulates podosome formation to facilitate cancer cell invasion. Scientific Reports, 7(1), 11523. https://doi.org/10.1038/S41598-017-11273-2 DOI: https://doi.org/10.1038/s41598-017-11273-2
Clapham, D. E. (2007). Calcium signaling. Cell, 131(6), 1047-1058. https://doi.org/10.1016/j.cell.2007.11.028 DOI: https://doi.org/10.1016/j.cell.2007.11.028
D’Amore, A., Hanbashi, A. A., Di-Agostino, S., Palombi, F., Sacconi, A., Voruganti, A., Taggi, M., Canipari, R., Blandino, G., Parrington, J., & Filippini, A. (2020). Loss of two-pore channel 2 (TPC2) expression increases the metastatic traits of melanoma cells by a mechanism involving the hippo signalling pathway and store-operated calcium entry. Cancers, 12(9), 2391. https://doi.org/10.3390/cancers12092391 DOI: https://doi.org/10.3390/cancers12092391
Daya, H. A., Kouba, S., Ouled-Haddou, H., Benzerdjeb, N., Telliez, M. S., Dayen, C., Sevestre, H., Garçon, L., Hague, F., & Ouadid-Ahidouch, H. (2021). Orai3-mediates cisplatin-resistance in non-small cell lung cancer cells by enriching cancer stem cell population through PI3K/AKT pathway. Cancers, 13(10), 2314. https://doi.org/10.3390/CANCERS13102314 DOI: https://doi.org/10.3390/cancers13102314
Di-Donato, M., Ostacolo, C., Giovannelli, P., Di Sarno, V., Monterrey, I. M. G., Campiglia, P., Migliaccio, A., Bertamino, A., & Castoria, G. (2021). Therapeutic potential of TRPM8 antagonists in prostate cancer. Scientific Reports, 11(1), 23232. https://doi.org/10.1038/s41598-021-02675-4 DOI: https://doi.org/10.1038/s41598-021-02675-4
Diez-Bello, R., Jardin, I., Lopez, J. J., El-Haouari, M., Ortega-Vidal, J., Altarejos, J., Salido, G. M., Salido, S., & Rosado, J. A. (2019). (−)-Oleocanthal inhibits proliferation and migration by modulating Ca2+ entry through TRPC6 in breast cancer cells. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research, 1866(3), 474-485. https://doi.org/10.1016/j.bbamcr.2018.10.010 DOI: https://doi.org/10.1016/j.bbamcr.2018.10.010
Dubois, C., Vanden-Abeele, F., Lehen’kyi, V., Gkika, D., Guarmit, B., Lepage, G., Slomianny, C., Borowiec, A. S., Bidaux, G., Benahmed, M., Shuba, Y., & Prevarskaya, N. (2014). Remodeling of channel-forming ORAI proteins determines an oncogenic switch in prostate cancer. Cancer Cell, 26(1), 19-32. https://doi.org/10.1016/J.CCR.2014.04.025 DOI: https://doi.org/10.1016/j.ccr.2014.04.025
Faouzi, M., Hague, F., Potier, M., Ahidouch, A., Sevestre, H., & Ouadid-Ahidouch, H. (2011). Downregulation of Orai3 arrests cell-cycle progression and induces apoptosis in breast cancer cells but not in normal breast epithelial cells. Journal of Cellular Physiology, 226(2), 542-551. https://doi.org/10.1002/jcp.22363 DOI: https://doi.org/10.1002/jcp.22363
Feng, M., Grice, D. M., Faddy, H. M., Nguyen, N., Leitch, S., Wang, Y., Muend, S., Kenny, P. A., Suku-mar, S., Roberts-Thomson, S. J., Monteith, G. R., & Rao, R. (2010). Store-independent activation of Orai1 by SPCA2 in mammary tumors. Cell, 143(1), 84-98. https://doi.org/10.1016/j.cell.2010.08.040 DOI: https://doi.org/10.1016/j.cell.2010.08.040
Gao, P., Peng, T., Lin, S., Zhi, W., Cao, C., Wu, P., Xi, L., Wu, P., Yang, Q., & Ding, W. (2021). Key role of MCUR1 in malignant progression of breast cancer. Onco Targets and Therapy, 14, 4163-4175. https://doi.org/10.2147/OTT.S306854 DOI: https://doi.org/10.2147/OTT.S306854
Ge, C., Zeng, B., Li, R., Li, Z., Fu, Q., Wang, W., Wang, Z., Dong, S., Lai, Z., Wang, Y., Xue, Y., Guo, J., Di, T., & Song, X. (2019). Knockdown of STIM1 expression inhibits non-small-cell lung cancer cell proliferation in vitro and in nude mouse xenografts. Bioengineered, 10(1), 425-436. https://doi.org/10.1080/21655979.2019.1669518 DOI: https://doi.org/10.1080/21655979.2019.1669518
Hanahan, D. & Weinberg, R. A. (2011). Hallmarks of cancer: the next generation. Cell, 144(5), 646-674. https://doi.org/10.1016/j.cell.2011.02.013 DOI: https://doi.org/10.1016/j.cell.2011.02.013
Humeau, J., Bravo-San-Pedro, J. M., Vitale, I., Nuñez, L., Villalobos, C., Kroemer, G., & Senovilla, L. (2018). Calcium signaling and cell cycle: Progression or death. Cell Calcium, 70, 3-15. https://doi.org/10.1016/j.ceca.2017.07.006 DOI: https://doi.org/10.1016/j.ceca.2017.07.006
Jardin, I., Diez-Bello, R., Falcon, D., Alvarado, S., Regodon, S., Salido, G. M., Smani, T., & Rosado, J. A. (2021). Melatonin downregulates TRPC6, impairing store-operated calcium entry in triple-negative breast cancer cells. Journal of Biological Chemistry, 296, 100254. https://doi.org/10.1074/jbc.RA120.015769 DOI: https://doi.org/10.1074/jbc.RA120.015769
Jin, M., Wang, J., Ji, X., Cao, H., Zhu, J., Chen, Y., Yang, J., Zhao, Z., Ren, T., & Xing, J. (2019). MCUR1 facilitates epithelial-mesenchymal transition and metastasis via the mitochondrial calcium dependent ROS/Nrf2/Notch pathway in hepatocellular carcinoma. Journal of Experimental & Clinical Cancer Research, 38(1), 136. https://doi.org/10.1186/S13046-019-1135-X DOI: https://doi.org/10.1186/s13046-019-1135-x
Jones, C. A. & Hazlehurst, L. A. (2021). Role of Calcium Homeostasis in Modulating EMT in Cancer. Biomedicines, 9(9), 1200. https://doi.org/10.3390/biomedicines9091200 DOI: https://doi.org/10.3390/biomedicines9091200
Jung, J., Cho, K., Naji, A. K., Clemons, K. N., Wong, C. O., Villanueva, M., Gregory, S., Karagas, N. E., Tan, L., Liang, H., Rousseau, M. A., Tomasevich, K. M., Sikora, A. G., Levental, I., van-der-Hoeven, D., Zhou, Y., Hancock, J. F., & Venkatachalam, K. (2019). HRAS-driven cancer cells are vulnerable to TRPML1 inhibition. EMBO Reports, 20(4), e46685. https://doi.org/10.15252/embr.201846685 DOI: https://doi.org/10.15252/embr.201846685
Karacicek, B., Erac, Y., & Tosun, M. (2019). Functional consequences of enhanced expression of STIM1 and Orai1 in Huh-7 hepatocellular carcinoma tumor-initiating cells. BMC Cancer, 19(1), 751. https://doi.org/10.1186/S12885-019-5947-Z DOI: https://doi.org/10.1186/s12885-019-5947-z
Lai, H. T., Canoy, R. J., Campanella, M., & Vassetzky, Y. (2022). Ca2+ Transportome and the interorganelle communication in hepatocellular carcinoma. Cells, 11(5), 815. https://doi.org/10.3390/cells11050815 DOI: https://doi.org/10.3390/cells11050815
Lan, X., Zhao, J., Song, C., Yuan, Q., & Liu, X. (2019). TRPM8 facilitates proliferation and immune evasion of esophageal cancer cells. Bioscience Reports, 39(10), BSR20191878. https://doi.org/10.1042/BSR20191878 DOI: https://doi.org/10.1042/BSR20191878
Lee, S. H., Rigas, N. K., Lee, C. R., Bang, A., Srikanth, S., Gwack, Y., Kang, M. K., Kim, R. H., Park, N. H., & Shin, K. H. (2016). Orai1 promotes tumor progression by enhancing cancer stemness via NFAT signaling in oral/oropharyngeal squamous cell carcinoma. Oncotarget, 7(28), 43239-43255. https://doi.org/10.18632/oncotarget.9755 DOI: https://doi.org/10.18632/oncotarget.9755
Lehen’kyi, V., Flourakis, M., Skryma, R., & Prevarskaya, N. (2007). TRPV6 channel controls prostate cancer cell proliferation via Ca(2+)/NFAT-dependent pathways. Oncogene, 26(52), 7380-7385. https://doi.org/10.1038/sj.onc.1210545 DOI: https://doi.org/10.1038/sj.onc.1210545
Li, R. F., Man, Q. W., Liu, J. Y., Zheng, Y. Y., Gao, X., & Liu, H. M. (2021). Overexpression of T-type calcium channel Cav3.1 in oral squamous cell carcinoma: association with proliferation and anti-apoptotic activity. Journal of Molecular Histology, 52(3), 511-520. https://doi.org/10.1007/S10735-020-09937-X DOI: https://doi.org/10.1007/s10735-020-09937-x
Lin, D. C., Zheng, S. Y., Zhang, Z. G., Luo, J. H., Zhu, Z. L., Li, L., Chen, L. S., Lin, X., Sham, J. S. K., Lin, M. J., & Zhou, R. X. (2021a). TRPC3 promotes tumorigenesis of gastric cancer via the CNB2/GSK3b/NFATc2 signaling pathway. Cancer Letters, 519, 211-225. https://doi.org/10.1016/J.CANLET.2021.07.038 DOI: https://doi.org/10.1016/j.canlet.2021.07.038
Lin, R., Bao, X., Wang, H., Zhu, S., Liu, Z., Chen, Q., Ai, K., & Shi, B. (2021b). TRPM2 promotes pancreatic cancer by PKC/MAPK pathway. Cell Death & Dsease, 12(6), 585. https://doi.org/10.1038/s41419-021-03856-9 DOI: https://doi.org/10.1038/s41419-021-03856-9
Liu, J., Chen, Y., Shuai, S., Ding, D., Li, R., & Luo, R. (2014). TRPM8 promotes aggressiveness of breast cancer cells by regulating EMT via activating AKT/GSK-3b pathway. Tumour Biology, 35(9), 8969-8977. https://doi.org/10.1007/S13277-014-2077-8 DOI: https://doi.org/10.1007/s13277-014-2077-8
Liu, L., Wu, N., Wang, Y., Zhang, X., Xia, B., Tang, J., Cai, J., Zhao, Z., Liao, Q., & Wang, J. (2019). TRPM7 promotes the epithelial-mesenchymal transition in ovarian cancer through the calcium-related PI3K / AKT oncogenic signaling. Journal of Experimental & Clinical Cancer Research, 38(1), 106. https://doi.org/10.1186/S13046-019-1061-Y DOI: https://doi.org/10.1186/s13046-019-1061-y
Liu, Y., Jin, M., Wang, Y., Zhu, J., Tan, R., Zhao, J., Ji, X., Jin, C., Jia, Y., Ren, T., & Xing, J. (2020). MCU-induced mitochondrial calcium uptake promotes mitochondrial biogenesis and colorectal cancer growth. Signal Transduction and Targeted Therapy, 5(1), 59. https://doi.org/10.1038/S41392-020-0155-5 DOI: https://doi.org/10.1038/s41392-020-0155-5
Marchi, S., Giorgi, C., Galluzzi, L., & Pinton, P. (2020). Ca2+ Fluxes and Cancer. Molecular Cell, 78(6), 1055-1069. https://doi.org/10.1016/j.molcel.2020.04.017 DOI: https://doi.org/10.1016/j.molcel.2020.04.017
Miao, Y., Wang, X., Lai, Y., Lin, W., Huang, Y., Yin, H., Hou, R., & Zhang, F. (2021). Mitochondrial calcium uniporter promotes cell proliferation and migration in esophageal cancer. Oncology Letters, 22(3), 686. https://doi.org/10.3892/OL.2021.12947 DOI: https://doi.org/10.3892/ol.2021.12947
Monet, M., Lehen’kyi, V., Gackiere, F., Firlej, V.,Vandenberghe, M., Roudbaraki, M., Gkika, D., Pourtier, A., Bidaux, G., Slomianny, C., Delcourt, P., Rassendren, F., Bergerat, J. P., Ceraline, J., Cabon, F., Humez, S., & Prevarskaya, N. (2010). Role of cationic channel TRPV2 in promoting prostate cancer migration and progression to androgen resistance. Cancer Research, 70(3), 1225-1235. DOI: https://doi.org/10.1158/0008-5472.CAN-09-2205
Monteith, G. R., Prevarskaya, N., & Roberts-Thomson, S. J. (2017). The calcium-cancer signalling nexus. Nature Reviews Cancer, 17(6), 367-380. https://doi.org/10.1038/nrc.2017.18 DOI: https://doi.org/10.1038/nrc.2017.18
Moon, D. O. (2023). Calcium’s role in orchestrating cancer apoptosis: Mitochondrial-centric perspective. International Journal of Molecular Sciences, 24(10), 8982. https://doi.org/10.3390/ijms24108982 DOI: https://doi.org/10.3390/ijms24108982
Morelli, M. B., Amantini, C., Tomassoni, D., Nabissi, M., Arcella, A., & Santoni, G. (2019). Transient Receptor Potential Mucolipin-1 Channels in Glioblastoma: Role in Patient’s Survival. Cancers, 11(4), 525. https://doi.org/10.3390/CANCERS11040525 DOI: https://doi.org/10.3390/cancers11040525
Morelli, M. B., Nabissi, M., Amantini, C., Tomassoni, D., Rossi, F., Cardinali, C., Santoni, M., Arcella, A., Oliva, M. A., Santoni, A., Polidori, C., Mariani, M. P., & Santoni, G. (2016). Overexpression of transient receptor potential mucolipin-2 ion channels in gliomas: role in tumor growth and progression. Oncotarget, 7(28), 43654. https://doi.org/10.18632/ONCOTARGET.9661 DOI: https://doi.org/10.18632/oncotarget.9661
Motiani, R. K., Hyzinski-García, M. C., Zhang, X., Henkel, M. M., Abdullaev, I. F., Kuo, Y. H., Matrougui, K., Mongin, A. A., & Trebak, M. (2013). STIM1 and Orai1 mediate CRAC channel activity and are essential for human glioblastoma invasion. Pflugers Archive: European Journal of Physiology, 465(9), 1249-1260. https://doi.org/10.1007/s00424-013-1254-8 DOI: https://doi.org/10.1007/s00424-013-1254-8
Müller, M., Gerndt, S., Chao, Y. K., Zisis, T., Nguyen, O. N. P., Gerwien, A., Urban, N., Müller, C., Gegenfurtner, F. A., Geisslinger, F., Ortler, C., Chen, C. C., Zahler, S., Biel, M., Schaefer, M., Grimm, C., Bracher, F., Vollmar, A. M., & Bartel, K. (2021). Gene editing and synthetically accessible inhibitors reveal role for TPC2 in HCC cell proliferation and tumor growth. Cell Chemical Biology, 28(8), 1119-1131.e27. https://doi.org/10.1016/j.chembiol.2021.01.023 DOI: https://doi.org/10.1016/j.chembiol.2021.01.023
Pan, Y., Huang, J., Liu, K., Xie, C., Chen, H., Guo, Z., Guo, S., & Chen, Y. (2022). Orai1-mediated store-operated Ca2+ entry promotes cervical cancer progression through IL-6 signaling. Frontiers in Molecular Biosciences, 9, 1041674. https://doi.org/10.3389/FMOLB.2022.1041674/BIBTEX DOI: https://doi.org/10.3389/fmolb.2022.1041674
Pathak, T., Gueguinou, M., Walter, V., Delierneux, C., Johnson, M. T., Zhang, X., Xin, P., Yoast, R. E., Emrich, S. M., Yochum, G. S., Sekler, I., Koltun, W. A., Gill, D. L., Hempel, N., & Trebak, M. (2020). Dichotomous role of the human mitochondrial Na+/Ca2+/Li+ exchanger NCLX in colorectal cancer growth and metastasis. eLife, 9, 1-41. https://doi.org/10.7554/eLife.59686 DOI: https://doi.org/10.7554/eLife.59686
Ren, T., Zhang, H., Wang, J., Zhu, J., Jin, M., Wu, Y., Guo, X., Ji, L., Huang, Q., Zhang, H., Yang, H., & Xing, J. (2017). MCU-dependent mitochondrial Ca2+ inhibits NAD+/SIRT3/SOD2 pathway to promote ROS production and metastasis of HCC cells. Oncogene, 36(42), 5897-5909. https://doi.org/10.1038/onc.2017.167 DOI: https://doi.org/10.1038/onc.2017.167
Revathidevi, S. & Munirajan, A. K. (2019). Akt in cancer: Mediator and more. Seminars in Cancer Biology, 59, 80-91. https://doi.org/10.1016/J.SEMCANCER.2019.06.002 DOI: https://doi.org/10.1016/j.semcancer.2019.06.002
Roderick, H. L. & Cook, S. J. (2008). Ca2+ signalling checkpoints in cancer: remodelling Ca2+ for cancer cell proliferation and survival. Nature Reviews Cancer, 8(5), 361-375. https://doi.org/10.1038/nrc2374 DOI: https://doi.org/10.1038/nrc2374
Sanchez-Collado, J., Lopez, J. J., Cantonero, C., Jardin, I., Regodón, S., Redondo, P. C., Gordillo, J., Smani, T., Salido, G. M., & Rosado, J. A. (2022). Orai2 modulates store-operated Ca2+ entry and cell cycle progression in breast cancer cells. Cancers, 14(1), 114. https://doi.org/10.3390/CANCERS14010114/S1 DOI: https://doi.org/10.3390/cancers14010114
Schaefer, E. A. M., Stohr, S., Meister, M., Aigner, A., Gudermann, T., & Buech, T. R. H. (2013). Stimulation of the chemosensory TRPA1 cation channel by volatile toxic substances promotes cell survival of small cell lung cancer cells. Biochemical Pharmacology, 85(3), 426-438. https://doi.org/10.1016/j.bcp.2012.11.019 DOI: https://doi.org/10.1016/j.bcp.2012.11.019
Singh, A. K., Roy, N. K., Bordoloi, D., Padmavathi, G., Banik, K., Khwairakpam, A. D., Kunnumakkara, A. B., & Sukumar, P. (2020). Orai-1 and Orai-2 regulate oral cancer cell migration and colonisation by suppressing Akt/mTOR/NF-kB signalling. Life Sciences, 261, 118372. https://doi.org/10.1016/J.LFS.2020.118372 DOI: https://doi.org/10.1016/j.lfs.2020.118372
Siow, W. X., Kabiri, Y., Tang, R., Chao, Y. K., Plesch, E., Eberhagen, C., Flenkenthaler, F., Fröhlich, T., Bracher, F., Grimm, C., Biel, M., Zischka, H., Vollmar, A. M., & Bartel, K. (2022). Lysosomal TRPML1 regulates mitochondrial function in hepatocellular carcinoma cells. Journal of Cell Science, 135(6), jcs259455. https://doi.org/10.1242/jcs.259455 DOI: https://doi.org/10.1242/jcs.259455
Sritangos, P., Pena Alarcon, E., James, A. D., Sultan, A., Richardson, D. A., & Bruce, J. I. E. (2020). Plasma membrane Ca2+ ATPase isoform 4 (PMCA4) has an important role in numerous hallmarks of pancreatic cancer. Cancers, 12(1), 1-22. https://doi.org/10.3390/cancers12010218 DOI: https://doi.org/10.3390/cancers12010218
Sun, J., Lu, F., He, H., Shen, J., Messina, J., Mathew, R., Wang, D., Sarnaik, A. A., Chang, W. C., Kim, M., Cheng, H., & Yang, S. (2014). STIM1- and Orai1-mediated Ca2+ oscillation orchestrates invadopodium formation and melanoma invasion. Journal of Cell Biology, 207(4), 535. https://doi.org/10.1083/JCB.201407082 DOI: https://doi.org/10.1083/jcb.201407082
Tajada, S. & Villalobos, C. (2020). Calcium permeable channels in cancer hallmarks. Frontiers in Pharmacology, 11, 968. https://doi.org/10.3389/FPHAR.2020.00968 DOI: https://doi.org/10.3389/fphar.2020.00968
Thebault, S., Flourakis, M., Vanoverberghe, K., Vandermoere, F., Roudbaraki, M., Lehen’kyi, V., Slomianny, C., Beck, B., Mariot, P., Bonnal, J. L., Mauroy, B., Shuba, Y., Capiod, T., Skryma, R., & Prevarskaya, N. (2006). Differential role of transient receptor potential channels in Ca2+ entry and proliferation of prostate cancer epithelial cells. Cancer Research, 66(4), 2038-2047. https://doi.org/10.1158/0008-5472.CAN-05-0376 DOI: https://doi.org/10.1158/0008-5472.CAN-05-0376
Umemura, M., Baljinnyam, E., Feske, S., De-Lo-renzo, M. S., Xie, L. H., Feng, X., Oda, K., Makino, A., Fujita, T., Yokoyama, U., Iwatsubo, M., Chen, S., Goydos, J. S., Ishikawa, Y., & Iwatsubo, K. (2014). Store-operated Ca2+ entry (SOCE) regulates melanoma proliferation and cell migration. PloS One, 9(2), e89292. https://doi.org/10.1371/JOURNAL.PONE.0089292 DOI: https://doi.org/10.1371/journal.pone.0089292
Wan, H., Gao, N., Lu, W., Lu, C., Chen, J., Wang, Y., & Dong, H. (2022). NCX1 coupled with TRPC1 to promote gastric cancer via Ca2+/AKT/b-catenin pathway. Oncogene, 41(35), 4169-4182. https://doi.org/10.1038/S41388-022-02412-9 DOI: https://doi.org/10.1038/s41388-022-02412-9
Wang, G., Cao, R., Qian, K., Peng, T., Yuan, L., Chen, L., Cheng, S., Xiong, Y., Ju, L., Wang, X., & Xiao, Y. (2020a). TRPM8 inhibition regulates the proliferation, migration and ROS metabolism of bladder cancer cells. Onco Targets and Therapy, 13, 8825-8835. https://doi.org/10.2147/OTT.S257056 DOI: https://doi.org/10.2147/OTT.S257056
Wang, J., Liao, Q. J., Zhang, Y., Zhou, H., Luo, C. H., Tang, J., Wang, Y., Tang, Y., Zhao, M., Zhao, X. H., Zhang, Q. Y., & Xiao, L. (2014). TRPM7 is required for ovarian cancer cell growth, migration and invasion. Biochemical and Biophysical Research Communications, 454(4), 547–553. https://doi.org/10.1016/J.BBRC.2014.10.118 DOI: https://doi.org/10.1016/j.bbrc.2014.10.118
Wang, T., Li, N., Jin, L., Qi, X., Zhang, C., & Hua, D. (2020b). The calcium pump PMCA4 prevents epithelial-mesenchymal transition by inhibiting NFATc1-ZEB1 pathway in gastric cancer. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research, 1867(12), 118833. https://doi.org/10.1016/J.BBAMCR.2020.118833 DOI: https://doi.org/10.1016/j.bbamcr.2020.118833
Wang, X., Li, Y., Li, Z., Lin, S., Wang, H., Sun, J., Lan, C., Wu, L., Sun, D., Huang, C., Singh, P. K., Hempel, N., Trebak, M., De-Nicola, G. M., Hao, J., & Yang, S. (2022). Mitochondrial calcium uniporter drives metastasis and confers a targetable cystine dependency in pancreatic cancer. Cancer Research, 82(12), 2254-2268. https://doi.org/10.1158/0008-5472.CAN-21-3230 DOI: https://doi.org/10.1158/0008-5472.CAN-21-3230
Wang, X., Song, X., Cheng, G., Zhang, J., Dong, L., Bai, J., Luo, D., Xiong, Y., Li, S., Liu, F., Sun, Y., Wang, X., Li, Y., & Huang, Y. (2020c). The regulatory mechanism and biological gignificance of mitochondrial calcium uniporter in the migration, invasion, angiogenesis and growth of gastric cancer. Onco Targets and Therapy, 13, 11781-11794.https://doi.org/10.2147/OTT.S262049 DOI: https://doi.org/10.2147/OTT.S262049
Wang, Y., Wang, H., Pan, T., Li, L., Li, J., & Yang, H. (2017). STIM1 silencing inhibits the migration and invasion of A549 cells. Molecular Medicine Reports, 16(3), 3283-3289. https://doi.org/10.3892/MMR.2017.7010 DOI: https://doi.org/10.3892/mmr.2017.7010
Xia, J., Wang, H., Huang, H., Sun, L., Dong, S., Huang, N., Shi, M., Bin, J., Liao, Y., & Liao, W. (2016). Elevated Orai1 and STIM1 expressions upregulate MACC1 expression to promote tumor cell proliferation, metabolism, migration, and invasion in human gastric cancer. Cancer Letters, 381(1), 31-40. https://doi.org/10.1016/j.canlet.2016.07.014 DOI: https://doi.org/10.1016/j.canlet.2016.07.014
Xu, S., Cheng, X., Wu, L., Zheng, J., Wang, X., Wu, J., Yu, H., Bao, J., & Zhang, L. (2020). Capsaicin induces mitochondrial dysfunction and apoptosis in anaplastic thyroid carcinoma cells via TRPV1-mediated mitochondrial calcium overload. Cellular Signalling, 75, 109733. https://doi.org/10.1016/j.cellsig.2020.109733 DOI: https://doi.org/10.1016/j.cellsig.2020.109733
Yang, S., Zhang, J. J., & Huang, X. Y. (2009). Orai1 and STIM1 are critical for breast tumor cell migration and metastasis. Cancer Cell, 15(2), 124-134. https://doi.org/10.1016/j.ccr.2008.12.019 DOI: https://doi.org/10.1016/j.ccr.2008.12.019
Zhang, L., Au-Yeung, C. L., Huang, C., Yeung, T. L., Ferri-Borgogno, S., Lawson, B. C., Kwan, S. Y., Yin, Z., Wong, S. T., Thomas, V., Lu, K. H., Yip, K. P., Sham, J. S. K., & Mok, S. C. (2022b). Ryanodine receptor 1-mediated Ca2+ signaling and mitochondrial reprogramming modulate uterine serous cancer malignant phenotypes. Journal of Experimental & Clinical Cancer Research, 41(1), 242. https://doi.org/10.1186/S13046-022-02419-W DOI: https://doi.org/10.21203/rs.3.rs-961544/v1
Zhang, L. Y., Zhang, Y. Q., Zeng, Y. Z., Zhu, J. L., Chen, H., Wei, X. L., & Liu, L. J. (2020). TRPC1 inhibits the proliferation and migration of estrogen receptor-positive Breast cancer and gives a better prognosis by inhibiting the PI3K/AKT pathway. Breast Cancer Research and Treatment, 182(1), 21-33. https://doi.org/10.1007/S10549-020-05673-8/FIGURES/7 DOI: https://doi.org/10.1007/s10549-020-05673-8
Zhang, P., Li, K., Wang, Z., Wu, Y., Zhang, H., Ma, F., Liu, X. Y., Tong, M. C. F., Ru, X., Zhang, X., & Zeng, X. (2022a). Transient receptor potential vanilloid type 4 (TRPV4) promotes tumorigenesis via NFAT4 activation in nasopharyngeal carcinoma. Frontiers in Molecular Biosciences, 9, 1064366. https://doi.org/10.3389/FMOLB.2022.1064366/BIBTEX DOI: https://doi.org/10.3389/fmolb.2022.1064366
Zhao, H., Yan, G., Zheng, L., Zhou, Y., Sheng, H., Wu, L., Zhang, Q., Lei, J., Zhang, J., Xin, R., Jiang, L., Zhang, X., Chen, Y., Wang, J., Xu, Y., Li, D., & Li, Y. (2020). STIM1 is a metabolic checkpoint regulating the invasion and metastasis of hepatocellular carcinoma. Theranostics, 10(14), 6483-6499. https://doi.org/10.7150/THNO.44025 DOI: https://doi.org/10.7150/thno.44025
Zhao, Y., Wang, J., & Liu, X. (2021). TRPV4 induces apoptosis via p38 MAPK in human lung cancer cells. Brazilian Journal of Medical and Biological Research, 54(12), e10867. https://doi.org/10.1590/1414-431X2021E10867 DOI: https://doi.org/10.1590/1414-431x2021e10867
Zhou, Y., Gu, P., Li, J., Li, F., Zhu, J., Gao, P., Zang, Y., Wang, Y., Shan, Y., & Yang, D. (2017). Suppression of STIM1 inhibits the migration and invasion of human prostate cancer cells and is associated with PI3K/Akt signaling inactivation. Oncology Reports, 38(5), 2629-2636. https://doi.org/10.3892/OR.2017.5961 DOI: https://doi.org/10.3892/or.2017.5961
Publicado
Cómo citar
Número
Sección
Categorías
Licencia
Derechos de autor 2024 Universidad Autónoma de Tamaulipas

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-CompartirIgual 4.0.
Aceptado 2024-08-22
Publicado 2024-09-09










